Describe the Size of an Atom
An atom is larger than a sheet of aluminum foil. Give an order of magnitude estimate of the size of an oxygen atom.
Describe the size and scale of an atom.
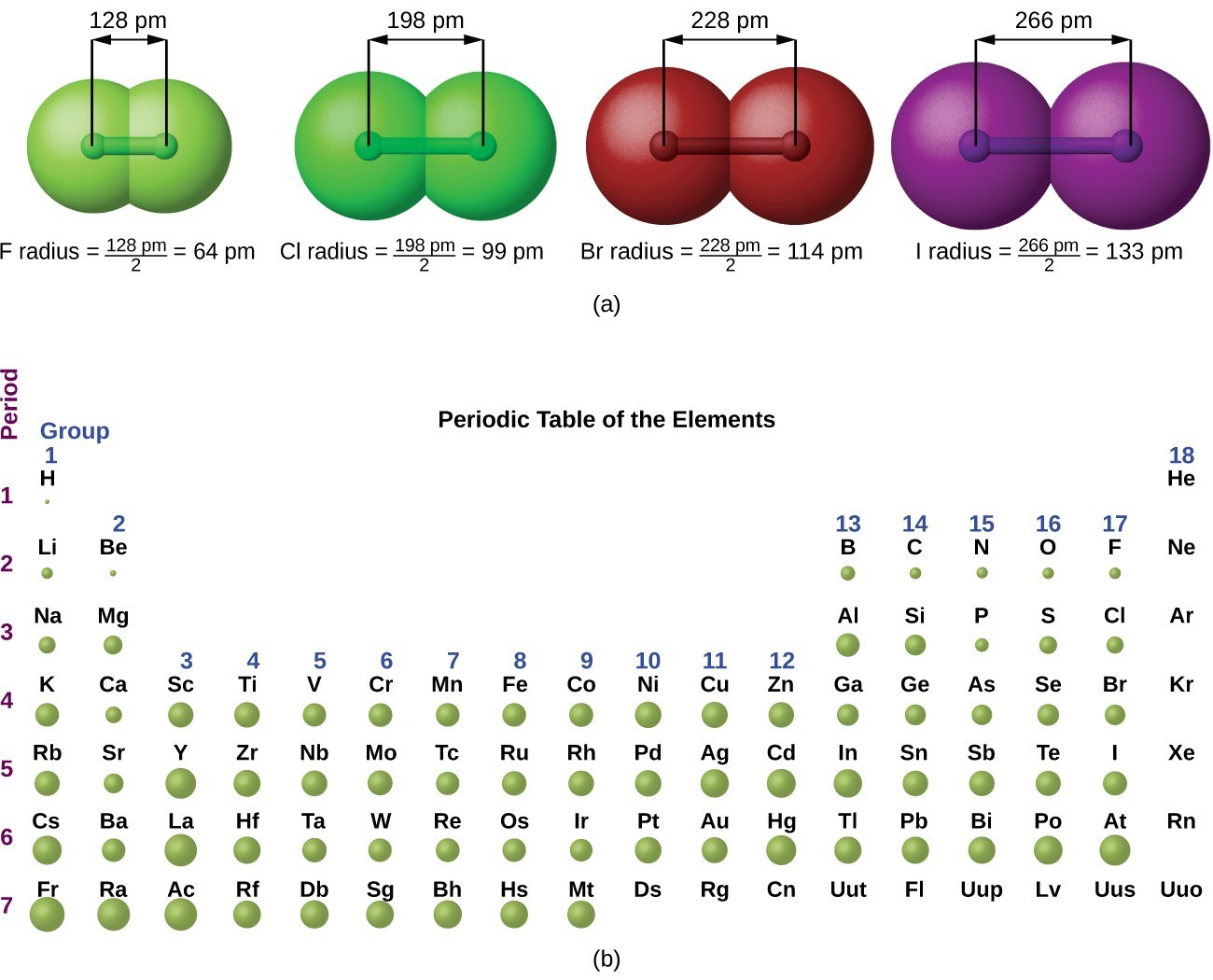
. Klemol 59 1 year ago. Atomic radiusA measure of the size of an atom. A substance made of only one type of atom is called an element.
The size of an atom can get defined by the situation. It represents the mean distance from the nucleus to the boundary of the surrounding cloud of electrons. Students will be able to explain that.
Size of a nucleus. The volume of the atom is 2 x 1013 times larger than its nucleus. Bohr radius - Wikipedia.
Neutrons are uncharged particles found within the nucleus. 10 10-11 10 10-10 m. This is due to the fact that electrons are tightly held in smaller atoms whereas in large atoms electrons are held quite loose ie lesser energy is required for removal of electrons from larger atoms than the smaller one.
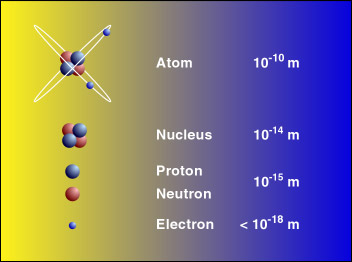
The nucleus of an atom is less than frac110000 the size of an atom. Describe the size content and the density of an atoms nucleus. This idea languished for about 2000 years until scientists in the 19th and 20thcenturies.
How would you describe the Structure of an Atom November 30 2020 by sastry After the discovery of electron and proton the scientists started thinking of arranging these particles in an atom. An atom is small but can be seen with just our eyes. Explain the relative of the nucleus in relation to the size of an entire atom.
An atom is tiny and cannot be seen without magnification. What is the approximate size of an atom. It is the only way to measure an atom because the orbital is the outer part of the atom and determines its size.
This means that if the nucleus were a typical pea then the atom would be about the size of a baseball field. As you can see the nucleus is significantly smaller than the atom which is quite superb if you ask me. Thomson was the first scientist to propose a.
Size of a nucleus. He proposed that there were in fact tiny indivisible pieces of matter that he called atomos meaning not to be cut. A good comparison of the nucleus to the atom is just like a pea in the midst of a track.
B For bonding you want the distanceradius nucleus to nucleus. Describe the size and scale of an atom. Get the latest describe the size of an atom news delivered straight to you.
Each electron has a negative charge -1 equal to the positive charge of a proton 1. Assuming atoms have a spherical shape the radius of the sphere describes the size of the atom. How could you describe the size of an atom.
A For spectrum you want the radius of each subshell. Get the latest describe the size of an atom news delivered straight to you. Protons and neutrons have approximately the same mass about 167 10-24 grams which scientists define as one atomic mass unit amu or one Dalton.
This means it is about 10-5 or 1100000 of the size of the whole atom. D It is the size of an apple or a large piece of fruit. An atom of oxygen has a diameter of 74 x 10-11 m.
Atoms exist but are very small and there are different types. Get an answer for describe how the size changes when an atom forms a cation and when an atom forms an anion and find homework help for other Science questions at eNotes. The atomic radius of a chemical element is a measure of the size of its atoms.
74 rounds up to 10. A good comparison of the nucleus to the atom is like a pea in the middle of a racetrack. Size of an atom.
Describe on Size of Atom. An atom is the size of a plastic building block. C It is the size of a grain of sand and can be seen with our eyes.
In simple terms an atom is a cloud of tiny electrons buzzing round a central much larger nucleus in a series of orbits called shells. The Hydrogen atom has a distance between the nucleus and the electron of. A It is so large it is bigger than a tree in the forest.
Size of atoms Atoms have a radius of about 01 nm nanometres or 00000000001 metres 1 10 -10 m. B It is so small it cannot be seen without magnification. The size of the nucleus of an atom is about 10 raise to power 15 meter which basically proposes that it is about 10 raise to power -5 or 1100000 we can say of the size of the entire atom.
Explain the relative size of the nucleus in relation to the size of the entire atom. Is measuring the size of an atom by the size of its orbital not very accurate. More than 2400 years ago the Greek philosopher Democritus began thinking about how many times matter could be divided.
As you can see the nucleus is significantly smaller than the atom which is quite superb. Size of an atom. The atom itself is about 50000 times larger than the nucleus a proton.
The nucleus of an atom is about 10-15 m in size. The larger the size of atom lesser is the ionization energy. An atom is the smallest particle that can create a chemical composition.
They begin with a thought experiment about size leading to the idea that atoms are extraordinarily small but they do exist. The ionization energy decreases with the increasing size of atom. Atoms vary by size but consider a hydrogen atom.

Eufisica The Evolution Concept Of The Atom Evolution Atom Science Nature
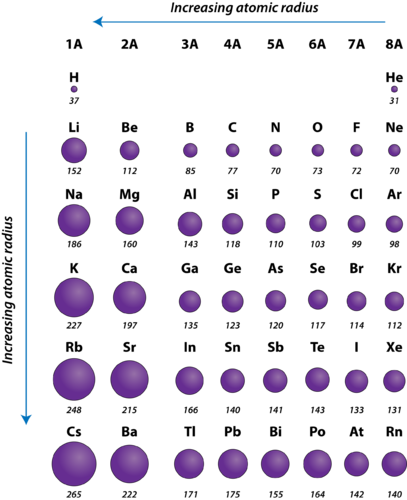
Periodic Trends Atomic Radius Chemistry For Non Majors

Boron By Carlos Clarivan Atomic Structure Boron Chemistry Worksheets

Models Of The Atom Timeline Youtube Atomic Theory Chemistry Chemistry Revision

Trends In The Periodic Table Chpt 7 1 Atomic Radius Size 2 Ionization Energy 3 Electronegativity The Th Ionization Energy Periodic Table Ionic Bonding

Pin On Caution Science Crossing

Atomic Size Introduction To Chemistry

The Atomic Radius Is In Shown In The Middle Of This Chart At 143 In Group 3a Teaching Chemistry Chemistry Lessons Chemistry Worksheets

Periodic Property Size Of The Atom Atomic Radius Science Chemistry Atom Chemistry

Mcdonald Publishing Atoms Elements Molecules Compounds Poster Set In 2022 Chemistry Education Teaching Chemistry Chemistry Classroom

The Size Of Atom Decreases Across A Period From Left To Right Description From Streamscience Blogspot Ionization Energy Chemistry Chemistry Periodic Table
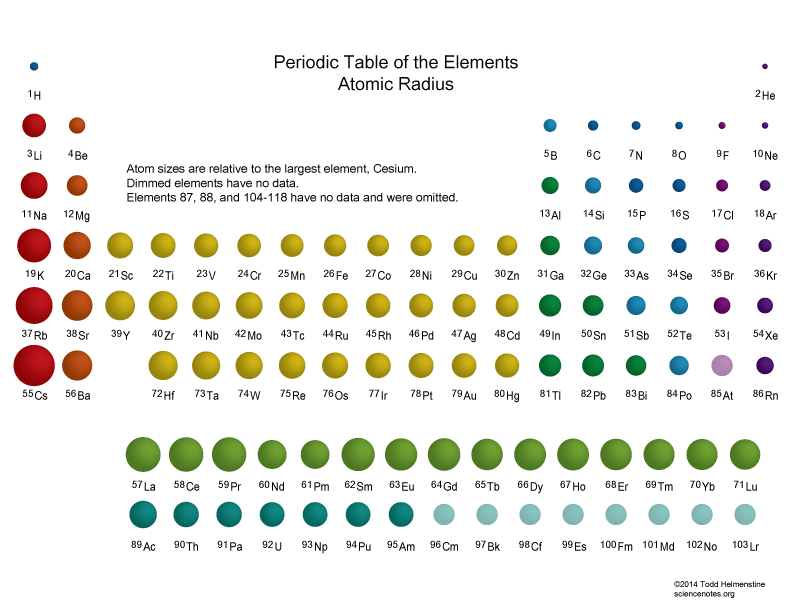
Which Are The Smallest And Largest Atoms Socratic

See The Electron Configuration Diagrams For Atoms Of The Elements Potassium Atom Electron Configuration Atom Diagram
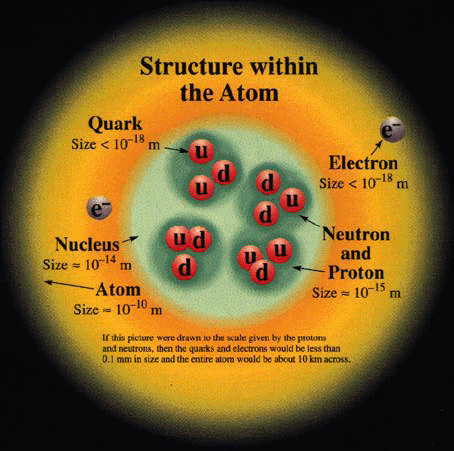



Comments
Post a Comment